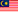Online Training on Good Distribution Practice for Medical Devices (GDPMD).
Cyberjaya, 15 December 2021 – Medical Device Authority Malaysia (MDA) has organised two days online training on Good Distribution Practice for Medical Devices (GDPMD).

“Our goals of the training are to provide an overview and interpretation of the clauses of GDPMD and to ensure the participants understand the scope and use of GDPMD in meeting the requirements of Medical Device” said Salbiah bt Yaakop, Director of Policy Code and Standard, MDA.

MDA is also targeting the participants to understand on the procedures and supporting documents to be developed in pursuing GDPMD, the importance of GDPMD as well as to understand on implementation of GDPMD systems as part of daily operations. Through this training, the participants are surely will gain understanding and insights in establishing the GDPMD system in their own.
This training is open to anyone who wants to comprehend on medical device regulatory requirements, overview on Good Distribution Practice for Medical Devices (GDPMD) comprising organization and GDPMD Regulatory Compliance System, establishment responsibilities, resource management, supply chain and device specification and for surveillance and vigilance.
Participated by 60 attendees, Madam Ir. Shamila Ariaratnam, a freelance consultant, trainer, auditor and founder of Armamentarium Consulting and has 20 years of experience as an engineer starting from programming fire and gas systems as a Software Engineer, has been invited to share her experience as a speaker.