ADVERTISEMENT OF MEDICAL DEVICE
![]()
ADVERTISEMENT OF MEDICAL DEVICE
What is the definition of advertisement in the Evidence Act 1950 [Act 56]?
Any statement, pictorial representation, or design, by means of any document as defined under the Evidence Act 1950 [Act 56] or by any other means, which is intended or claimed, whether directly or indirectly, to promote the use or supply of anything related to medical devices. Advertisement includes an announcement of a public nature, whether for the sale or purchase of a medical device or constituting an invitation to participate in an activity, and is conveyed by or through any signage, image, or sound disseminated through any medium for advertising purposes.
Conclusion à Advertisement refers to any statement, pictorial representation, or design intended to promote the use or supply of medical devices, whether directly or indirectly, through any medium, including signage, images, or sound.
How can I submit an advertisement application for medical devices?
All information, guidelines, and guidance documents that clarify the requirements for advertising medical devices that require approval or do not require approval can be referred to;
Ø Guidance Documents MDA/GD/0032: Code of Advertisement
Ø Guidelines: Application for Medical Device Advertisement Approval - Requirements
Ø Advertisement Application Form
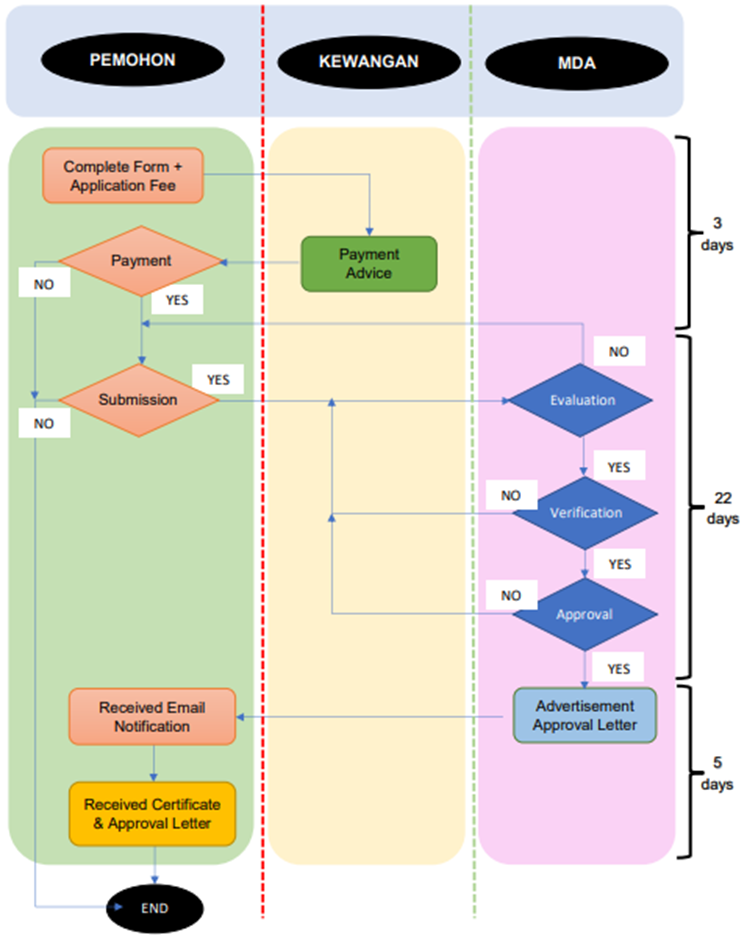
The process flow for an advertisement application is as follows:
Submission and Payment Instructions for Medical Device Advertising
IMPORTANCE NOTICE:
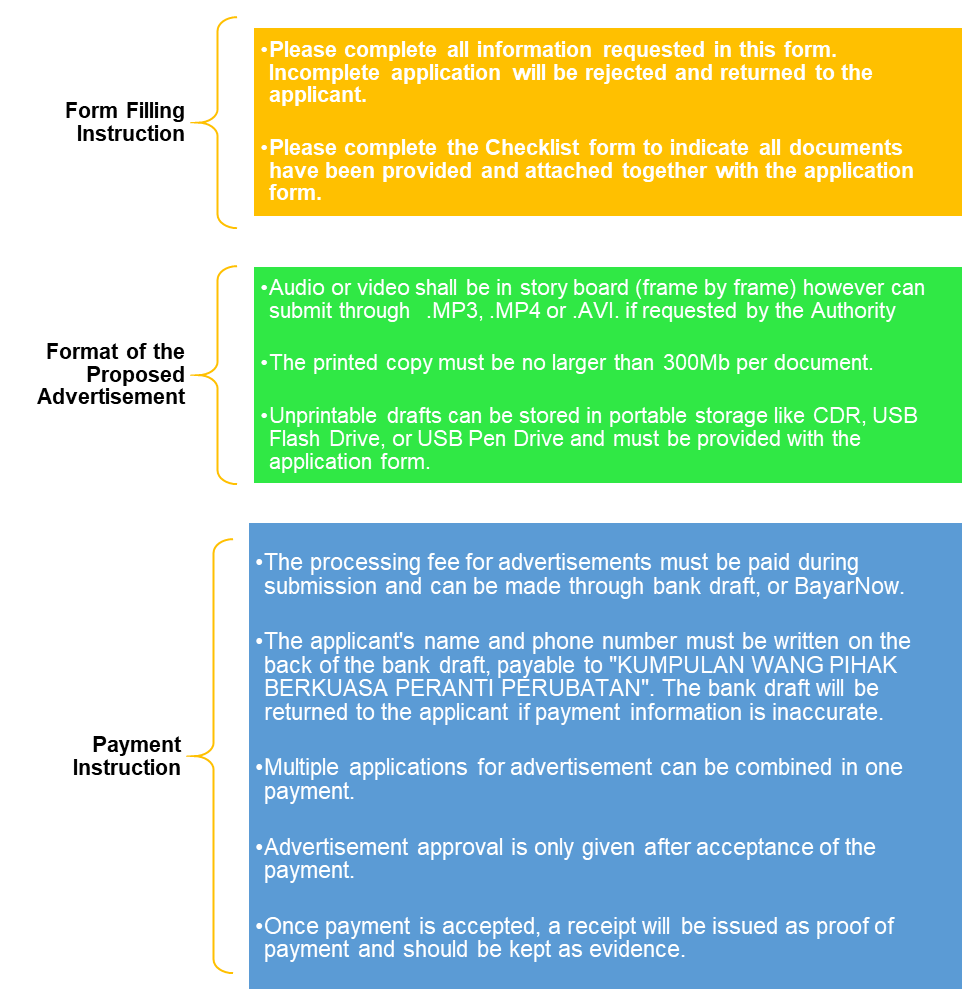
The form requires applicants to complete all requested information, complete the Checklist form to confirm all documents have been provided, and send your application via email to [email protected], as incomplete applications will be rejected.
Please take note that commencing 1st December 2023, all submissions for advertisement are required to be sent via email to [email protected]. Any applications received via hardcopy after this date will not be processed.
For any inquiries and further information;
v Email to [email protected]
v Officer in-charge;
(1) Nur Syafura +603-8230 0352
(2) Suhaida Rasul +603-8230 0214
Updated: 7 November 2023



