CONFORMITY ASSESSMENT BODY (CAB)
Definition: A Conformity Assessment Body (CAB) is an organization registered and recognized by Medical Device Authority (MDA) under the Medical Device Act 2012 (Act 737) in Malaysia. The CAB plays an important role in the regulatory framework for medical devices by conducting various conformity assessments to ensure that medical devices comply with the applicable standards, regulations, or requirements. The conformity assessments conducted by CABs may cover various areas, including product safety, performance, and quality management systems. The ultimate goal is to ensure that medical devices placed on the market are safe and perform as intended by the manufacturer and, therefore conforms to essential principles of safety and performance for medical devices.
Guidance Documents: Attachment 1
Online Application for CAB Registration: MeDC@St 2.0
Application Checklist for CAB Registration: Attachment 2
How to Create MeDC@St Account: Attachment 3
Proficiency Training Types: Attachment 4
Proficiency Training Calendar: Attachment 5
Proficiency Training Syllabus: Attachment 6
List of Registered Conformity Assessment Bodies: Attachment 7
List of Registered Auditors and Technical Personnel: Attachment 8
List of Technical Expert: Attachment 9
Frequently Ask Questions (FAQs): Attachment 10
Any inquiries, please email to: [email protected]
Contact numbers of relevant officers:
(1) Mohd Fadhullah Bin Abd Halim (Mr.) | Tel: +603-8230 0356 | E-mail: [email protected]
(2) Remee Binti Awang Jalil (Pn.) | Tel: +603-8230 0372 | E-mail: [email protected]
(3) Muhamad Sahafie Bin Zainal (Mr.) | Tel: +603-8230 0361 | E-mail: [email protected]
Process flow:
Registration of CAB, Auditors and Technical Personnel
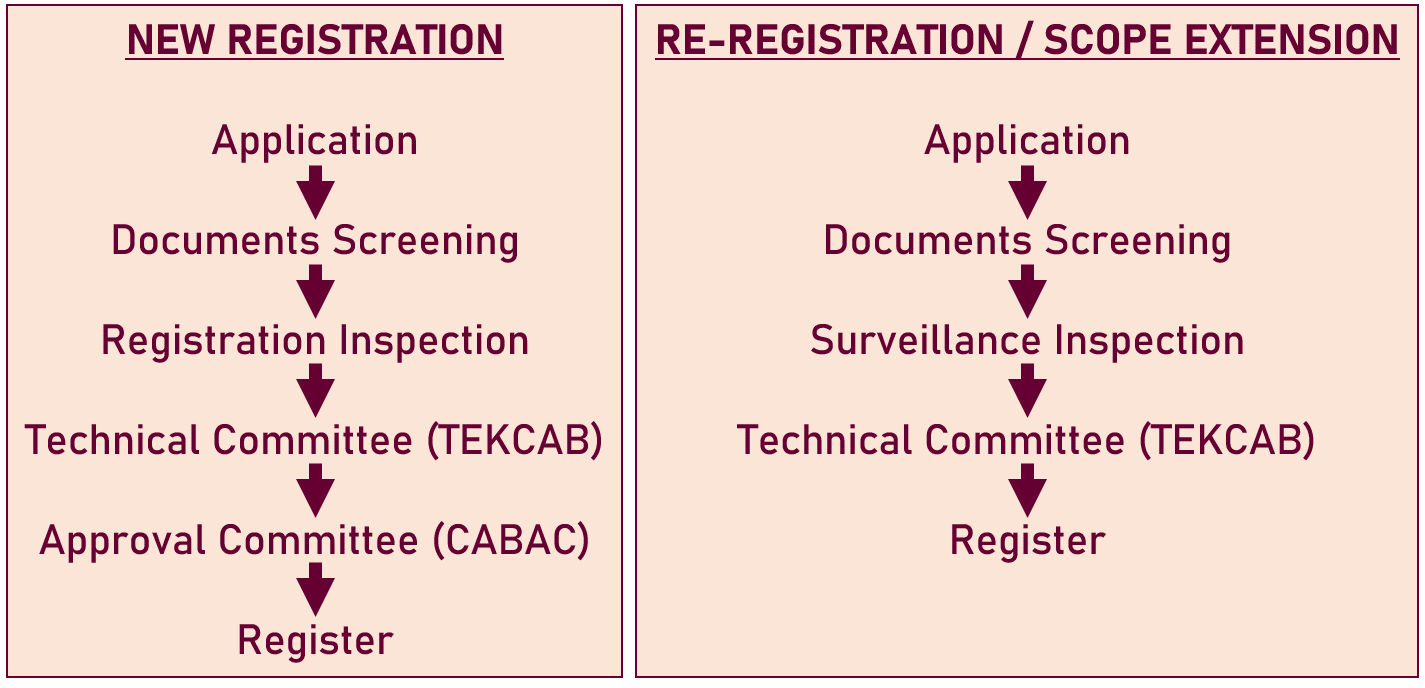
Important Notices:
[For new organization seeking to become Conformity Assessment Body]
- Prospective Conformity Assessment Bodies (CABs) are invited to carefully review and follow the outlined application procedures and stipulated requirements, which are available for review through this website page.
- Prospective CABs are expected to uphold principles of independence and impartiality throughout their operations. Avoiding conflicts of interest and maintaining objectivity during conformity assessments are critical.
- Prospective CABs are required to establish and maintain a robust Quality Management System (QMS) in accordance with MDA/GD/0023.
- Prospective CABs should be aware of the expected timeline for the evaluation of their applications. Information on the evaluation process, including potential site visits or inspections, is available for review through this website page.
Updated: 5 January 2024



