Custom-made Medical Device
|
Announcement: Clarification on the regulation of Dental Products The Medical Device Authority (MDA) wishes to clarify that dental products, including but not limited to crowns and aligners, are medical devices that fall under the regulatory requirements of the Medical Device Act 2012 [Act 737]. Accordingly, all local companies including laboratories involved in the manufacturing of such dental products must ensure that these devices are registered with the Authority to comply with Act 737. In cases where the dental products meet the criteria for custom-made medical devices, manufacturers may submit an exemption application directly to the Authority. This exemption is applicable only when the specific requirements of a custom-made device are fulfilled. This announcement serves to reinforce the importance of regulatory compliance and to support the safe and effective use of dental products in Malaysia. Date of published: 11-Nov-2024 |
Custom made Criteria :
1. Designed and manufactured according to a written prescription from a qualified medical practitioner for the sole use of a particular patient.
2. Does not fall into the category of mass-produced medical devices that require adaptation for specific professional user requirements.
** Medcast form requirement: - Crucial to adhere (click here) -
How to apply?
A. Notification form : Medcast
B. How to create Medcast Account : Medcast – Notification Creation
C. Administrative Charge : RM 300
D. Guidance Documents : NOTIFICATION OF CUSTOM-MADE (MDA/GD/0064)
E. Any inquiries, please email to sa.cm@mda.gov.my
F. Contact No. relevant Officer:
- Puan Haidar Nadirah Bt Hawalig +603-8230 0250
- Puan Nur Maizura Bt Zarmani +603-8230 0339
- En. Mohamad Aznil bin Ahmad Azmi (Printing Officer) +603-8230 0247
Process flow:
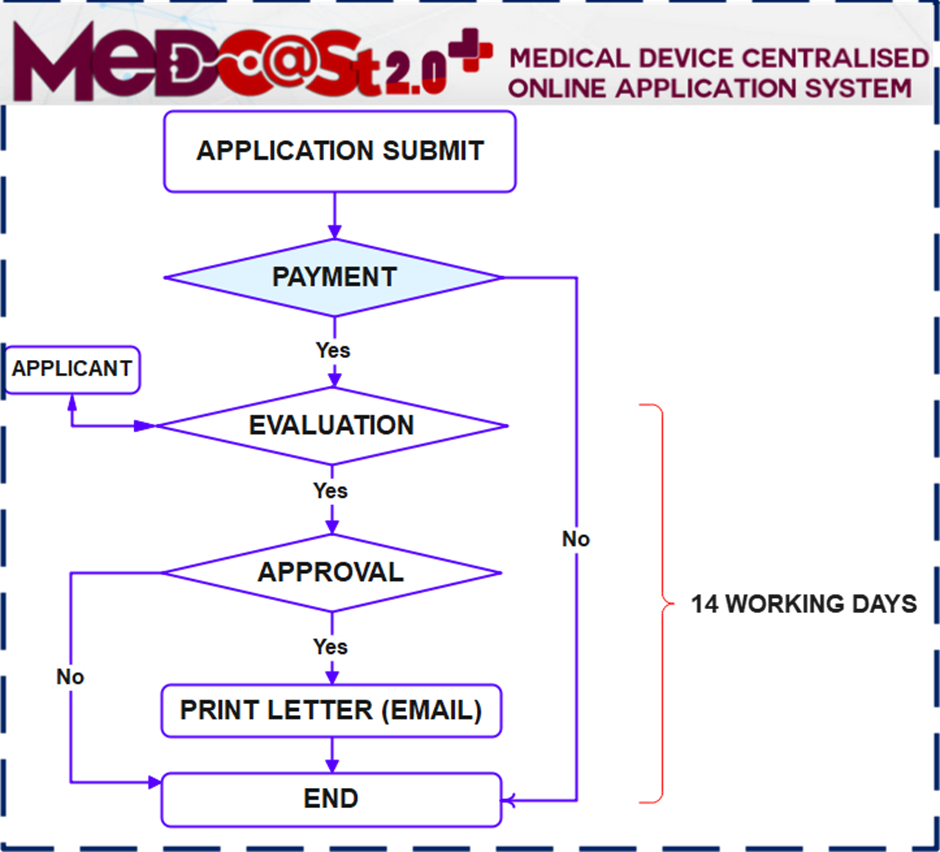
Importance Notice :
- Kindly note that commencing 1 December 2023, all submissions for Custom Made Medical Device Notifications are required to be made through the MeDC@St 2.0 online platform. The application processing period is 14 working days upon receipt of a complete application.
- The Custom-Made Notification System is activated using earlier version of Medcast 2.0. Users may face system bugs, but rest assured, it won't impact your application's submission. Applicants are advised to refer the Medcast Form Requirement to prevent any potential issues. If you encounter unresolved issues, please reach out to the MDA officer for assistance.
Updated: 8th October 2025






