Personal Use Medical Device
A. Introduction
Importation and placement of a medical device in the Malaysian market requires the medical device to comply with the requirements of the Medical Device Act 2012 (Act 737), and the medical device shall be registered with the Medical Device Authority (MDA) under Section 5 of Act 737. However, the Medical Device (Exemption) Order 2024 has provided an exemption from medical device registration and establishment license requirements for importation of personal use medical devices.
B. Scope of Exemption
The exemption applicable to personal use medical devices under the Medical Device (Exemption) Order 2024 are as follows:
1. Exemption from registration of medical devices
According to the Medical Device (Exemption) Order 2024, medical devices for the purpose of personal use have been exempted from the requirement for registration of medical devices under Section 5 of Act 737.
2. Exemption from establishment license
A person who imports medical devices for the purpose of personal use is exempted from the requirement of an establishment license under Section 15 of Act 737.
C. Definition of Personal Use Medical Device
A medical device which is brought into Malaysia for the use of a particular individual only and not to be placed in the market or be used by a third party.
[SOURCE: Medical Device (Exemption) Order 2024
NOTE: Refers to Section 43 in the Act 737 for explanation on a third party.
D. Requirements for Importing or Purchasing Personal Use Medical Devices
1. Medical devices for personal use may be imported or purchased subject to the following requirements:
a) The medical device shall be for the use of immediate family, and not for commercial purposes, resale, and distribution.
NOTE: Personal use medical devices must be purchased by an individual (under their own name) and cannot be purchased by an establishment or organization
b) To provide a formal prescription or letter of recommendation on the professional use medical device(s) from a registered healthcare professional, upon request from the Authority.
NOTE: Certain types of medical devices may not require recommendation from healthcare professionals.
Refer Annex A for examples.
c) for medical devices shall be appropriate according to the type of the medical device and there are a few examples as specified in Attachment 1 as reference.
NOTE:
Low/medium risk medical device (eg: Glucose Monitoring, Hearing Aids, In-vitro diagnostic products) : 1 piece of each type.
Consumable medical device (eg: contact lens, In-vitro maintenance test strips ) : Quantity for 3 months supply.
d) all labels and labelling information that comes with the medical device shall be retained; and
e) no same or similar medical device(s) is/are registered in Malaysia
NOTE: Please refer to Medical Device Authority Register (MDAR) : https://mdar.mda.gov.my
2. The importation of medical devices for the purpose of personal use is also exempted from the requirements of import permit under Customs (Prohibitions of Imports) Order 2023.
E. Regulatory Framework for Importation of Personal Use Medical Device
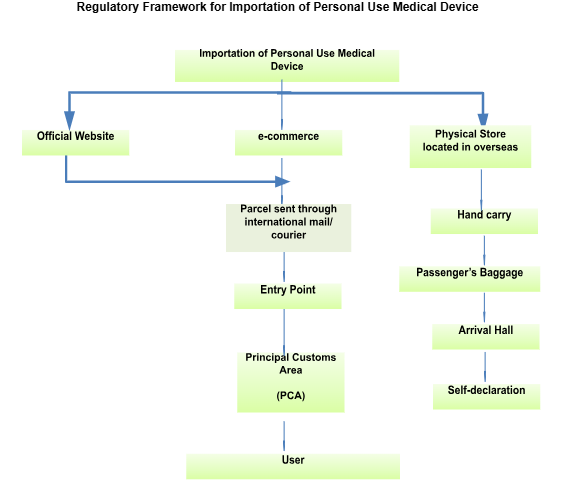
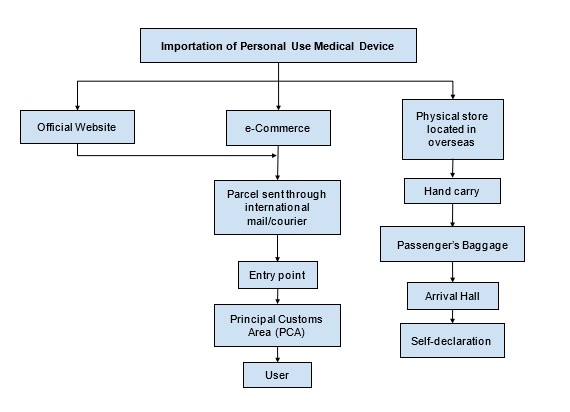
Any disputes or inquiries can be submitted via email to exemption.bhai@mda.gov.my or contact our representative, Pn. Nur Aisyah at 03 8230 0253
F. EXAMPLES AND SITUATIONS: Examples and Situations of Personal Use Medical Device
Date of upload: 22 December 2025
Prepared by: Policy and Strategic Planning Division
Uploaded by: Corporate Communication Division






