ADVERTISEMENT OF MEDICAL DEVICE
ADVERTISEMENT OF MEDICAL DEVICE
What is the definition of advertisement in the Evidence Act 1950 [Act 56]?
Any statement, pictorial representation, or design, by means of any document as defined under the Evidence Act 1950 [Act 56] or by any other means, which is intended or claimed, whether directly or indirectly, to promote the use or supply of anything related to medical devices. Advertisement includes an announcement of a public nature, whether for the sale or purchase of a medical device or constituting an invitation to participate in an activity, and is conveyed by or through any signage, image, or sound disseminated through any medium for advertising purposes.
Conclusion: Advertisement refers to any statement, pictorial representation, or design intended to promote the use or supply of medical devices, whether directly or indirectly, through any medium, including signage, images, or sound.
How to Submit a Medical Device Advertisement Application?
To submit an advertisement application for medical devices, please refer to the following guidelines and resources that clarify the requirements for advertisements that require or do not require approval:
Reference Documents:
- Guidance Documents MDA/GD/0032: Code of Advertisement – Outlines the standards and principles for advertising medical devices.
- Guidelines: Application for Medical Device Advertisement Approval - Requirements – Details the approval process and necessary documentation.
Application Forms & Templates:
- Advertisement Application Online Form – Digital submission platform for advertisement approval.
- Advertisement Application Form – Printable version for manual submission.
- Attestation & Declaration Form – A required form to certify the accuracy and compliance of the advertisement application.
- Template for an Advertisement Letter of Authorisation – Standard template for authorisation letters related to advertisement applications.
The process flow for an advertisement application is as follows:
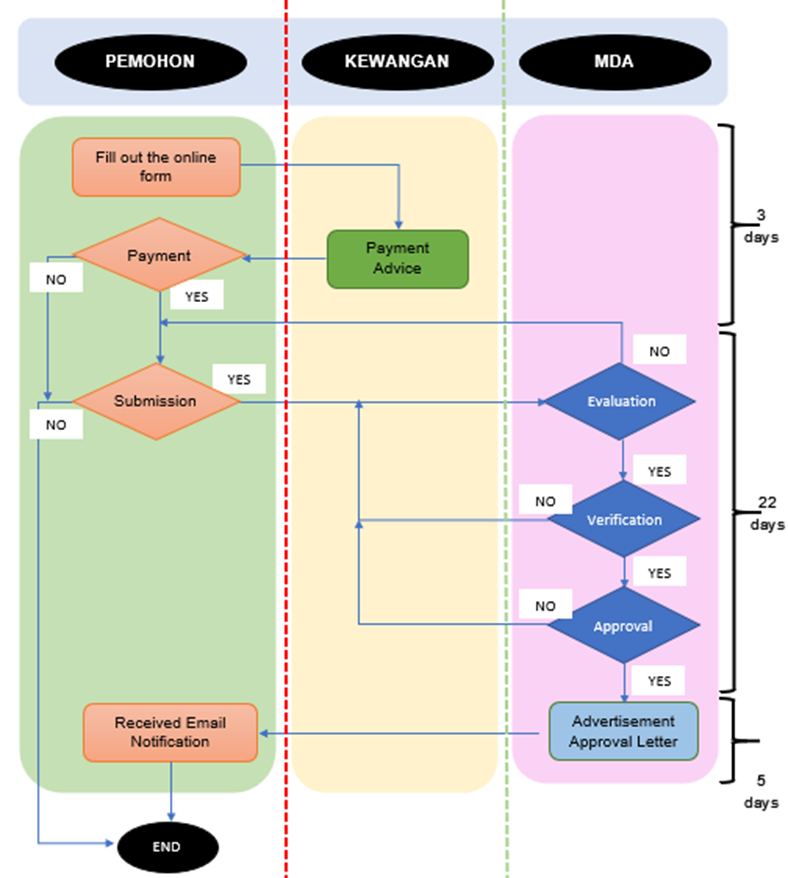
*working days
|
IMPORTANCE NOTICE: 1. Applicants are required to complete all requested information on the form to confirm that all necessary documents have been submitted. 2. Please ensure your application is submitted via the designated Google Form, as incomplete applications will be rejected. 3. Please note that from 1st March 2025, any application received via email will not be processed and will need to be re-applied using the online form. 4. This situation is subject to any terms and conditions that may apply. For any inquiries or further information, please contact: |
- Email to advertisement@mda.gov.my
- Officer in charge;
- Nur Maizura BInti Zarmani (03-82300 339)
- Hasdiana Binti Mohammadiah (03-82300 364)
Updated: 15th July 2025
By: Corporate Communication Division
Conditional Approval for Covid19 RTK (Self-Test)
![]()
CONDITIONAL APPROVAL FOR COVID-19 RTK (SELF-TEST)
- Guideline Document Conditional Approval for Covid-19 RTK (self-test)
- Form A - Application Form for Conditional Approval for Covid-19 RTK (Self-Test) *
- Form B - Evaluation Report Result Form for Conditional Approval for Covid-19 RTK (Self-Test) *
- Form C - Application Form For Conditional Approval For Self Test COVID-19 Test Kits (Lot To Lot Variation Test)
*Updated after 4 August consensus with Covid-19 Ivd Test Kit Special Access Evaluation Expert Committee Members
• E-mail: ca.covid19@mda.gov.my / Contact Number : +603 - 8230 0376
• In order to prevent email deliverability issues due to file size limitation, please submit the conditional approval application to ca.covid19@mda.gov.my
• Please use FORM A for submission of Conditional Approval for Covid-19 RTK (self-test) application. ( Send all of the softcopy supporting documents including Form A to email ca.covid19@mda.gov.my)
Additional Information on Conditional Approval for Covid-19 RTK (self-test)
|
No |
Description of fees for Conditional Approval for Covid-19 RTK (self-test) |
Fee Payable (RM) |
|
1 |
Application Fee |
150 |
|
2 |
Conditional Approval Letter Fee |
1000 |
*Updated after 4 August consensus with Covid-19 Ivd Test Kit Special Access Evaluation Expert Committee Members
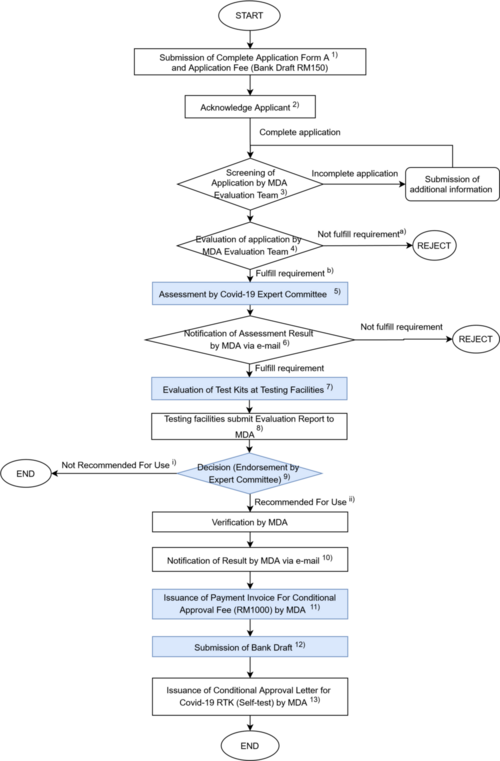
Instant Guide to Conditional Approval For Covid-19 RTK (self-test)
|
Step |
Explanatory Notes |
|
1 |
The applicant shall submit a complete Application Form (Form A) for Conditional Approval for Covid-19 RTK (self-test) together with the Application Fee RM 150 (Bank Draft). Note: Payment shall be made by Bank Draft to “KUMPULAN WANG PIHAK BERKUASA PERANTI PERUBATAN”. Please send the hardcopy of Bank Draft together with the form A to MDA. |
|
2 |
The Secretariat receiving the application will send an acknowledgement to the applicant. |
|
3 |
Screening of Application by MDA Evaluation Team and for incomplete applications it will be returned to the applicant for more information/documentation. |
|
4 |
The application will be evaluated using the Evaluation checklist (CRITERIA CHECKLIST FOR COVID-19 RTK (SELF-TEST) developed by MDA based on WHO and Recognized countries criteria) by the Evaluation team. a) Applications that do not meet the requirements will be rejected. b) Applications that meet the requirements will be moved to the Expert Committee (Jawatankuasa Pakar Penilaian Akses Khas Covid-19 IVD Test Kit) Meeting. |
|
5 |
The expert committee will review the content of each application against the checklist and the test kit that meet the requirements will be assigned to the testing facility. |
|
6 |
The Applicant will be notified of the Assessment Result for the application that meet the requirements via email together with an Evaluation Letter and Form B. |
|
7 |
The application that receives the evaluation letter shall go through evaluation testing at the designated assigned testing facility. |
|
8 |
MDA Evaluation Team will receive Evaluation Report from testing facilities. |
|
9 |
The test kits that meet the requirements for Recommended for Use will be endorsed during the meeting or by circulation based on the results of the evaluation report. i) Test kits that are not suggested for use will be refused and will not be permitted for usage, with an email notification. ii) Test kits that are recommended for use will be verified by Verification Officer (VO) |
|
10 |
The applicant will be notified of the decision through email. |
|
11 |
MDA Evaluation Team will issue a payment advice to the Finance Department (MDA) and the Finance Department will issue an invoice for Conditional Approval fee (RM 1,000) to the applicant. |
|
12 |
Submission of Bank Draft by applicant to Finance Department. Payment (Conditional Approval Fee) is accepted by the Finance Department, which notifies the Registration Unit. |
|
13 |
MDA will issue a Conditional Approval Letter for Covid-19 RTK (self-test). |
NOTIFICATION FOR EXPORT ONLY MEDICAL DEVICE
Notification of Export Only Medical Device
1. Introduction
- The importation, exportation, or placement of a medical device in the Malaysia market requires the medical device to be registered under Medical Device Act 2012 (Act 737). However, under Medical Device (Exemption) Order 2024 state that medical devices for purpose of export only are exempted from registration requirements and shall make an application for an exemption to the Authority.
- The exportation of unregistered medical device is allowed once the Export Only Medical Device Exemption Letter is issued by the Authority.
2. Requirements for Notification of Export Only Medical Device
- Applicant shall submit a notification before exportation of the first shipment.
- The application Export Only Medical Device Exemption shall be submitted via google form and the required documents are as follows:
|
No |
Documents
|
|
1 |
Registrar of Companies (ROC) certificate of applicant |
|
2 |
|
|
3 |
|
|
4 |
(Please refer to Figure 2: Determination whether LOA is required or not for Export Only Exemption) |
|
5 |
A copy manufacturer’s QMS ISO 13485 certificate |
|
6 |
A copy of Brochure/Leaflet/Label/IFU that contain information on brief description and intended use. |
|
7 |
A copy of establishment license (If applicable) |
Note: *new requirements for Notification of Export Only Medical Device
3. Determination whether LOA is required or not for Notification of Export Only Medical Device?
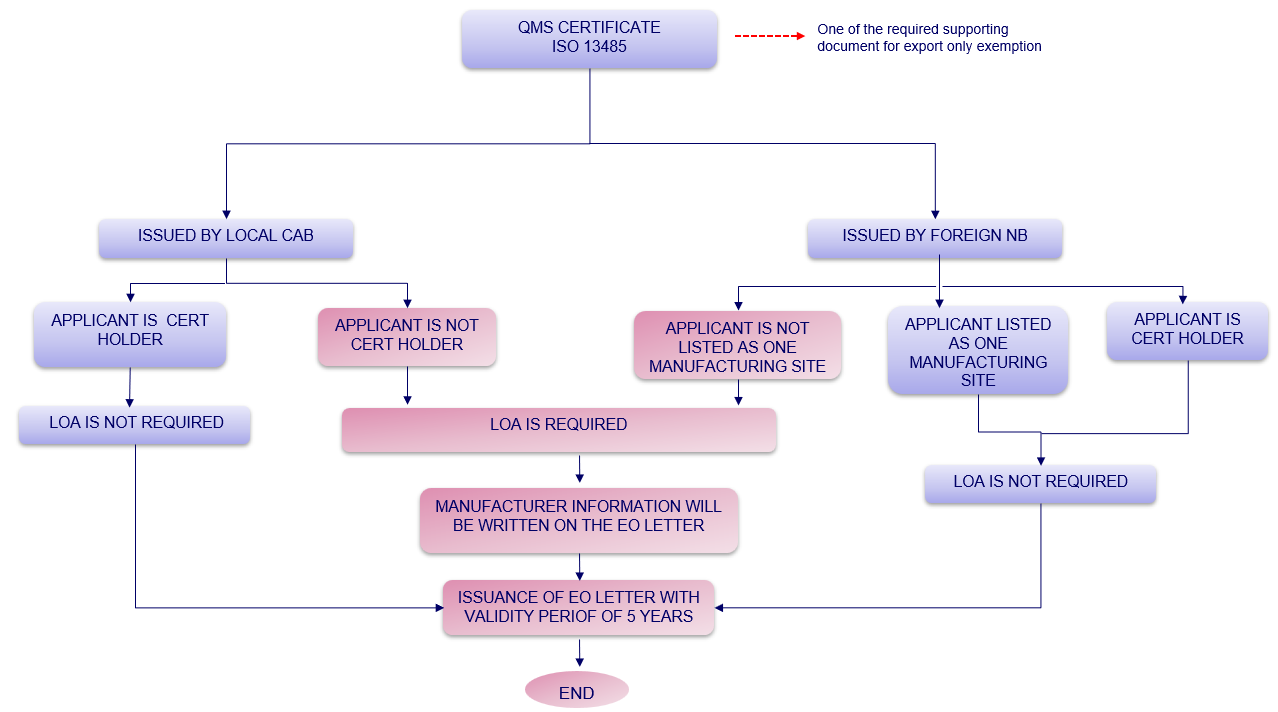
Figure 1: Determination whether LOA is required or not for Notification of Export Only Medical Device
4. Notification of Export Only Medical Device Process
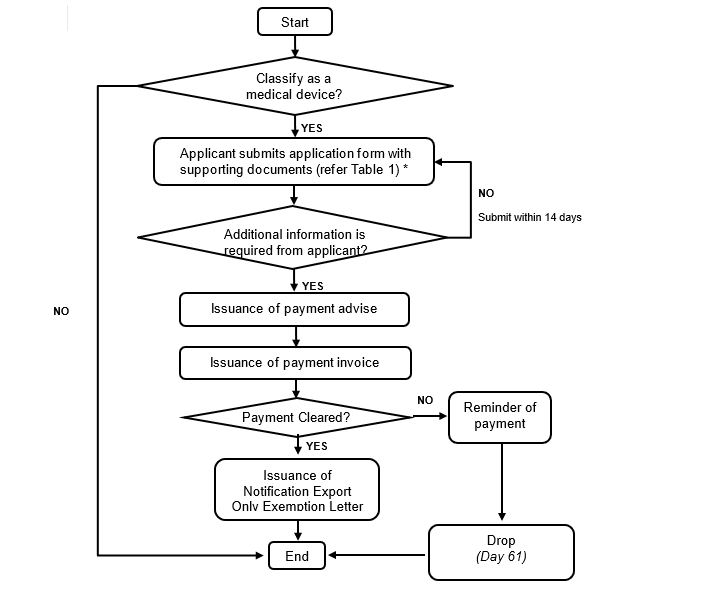
Figure 2: Notification of Export Only Medical Device Process
5. Fees for Notification of Export Only Medical Device
The payment RM500 shall be made online via portal BayarNow. For guidance on online payment is accessible via link or qr code below:

6. Issuance of Export Only Medical Device Exemption Letter
- Upon receipt of completed application and clearance of payment, the authority will issue an Export Only Medical Device Exemption Letter to the applicant within 14 days after clearance of payment, by letter and email.
- This Export Only Medical Device Exemption Letter permits multiple export consignments within the validity period of the letter.
- The validity period of the Export Only Medical Device Exemption Letter is five (5) years
- Once Export Only Medical Device Exemption Letter has expired or has been cancelled/withdrawn, no further export of the medical device, at any quantity, shall be permitted.
Any inquiries please email to exportonly.ec@mda.gov.my
General Line: +603 8230 0300
Direct Line : +603 8230 0253 (Pn. Nur Aisyah)
+603 8230 0208 (Pn. Hafizah)
Updated: 25 / 10 / 2023
List of User Manual and Guidance Document
|
No |
Title |
Download |
|
1. |
User Manual: i.Establishment Licence Application ii.Renewal of Establishment Licence Application iii. Amendment Major/Minor Application iv. Withdrawal Application |
|
|
2. |
User Manual for Change Of Ownership Application |
|
|
3. |
User Manual Video of Establishment Licence Application |
|
|
4. |
User Manual Video for Renewal of Establishment Licence Application |
|
|
5. |
User Manual Video of Amendment Major/Minor Application |
|
|
6. |
User Manual Video of Withdrawal Application |
|
|
7. |
User Manual Video of Change Of Ownership Application |
|
|
8. |
Guidance Document of Licensing for Establishment |
|
|
9. |
Guidance Document for Good Distribution Practice for Medical Devices (GDPMD) |
|
|
10. |
Guidance Document for Change Of Ownership Application |
|
Consultation
ANNOUNCEMENT ON MDA CONSULTANCY SERVICES
MDA offers regulatory consulting that can support stakeholders who are involve in regulatory submission for medical devices in Malaysia, to comply with medical device regulatory requirements under Medical Device Act 2012 (Act 737). The regulatory consulting services include:
Online consultancy maybe requested for applicant who isn’t physically able to meet MDA’s consultancy team. Consultancy *fee imposed is based on type of consultancy services selected.
*Note: Please refer to Consultancy Request Form (Package) and Consultancy Request Form (Non-Package)
The following diagram describes the step-by-step process of requesting our consultancy services:
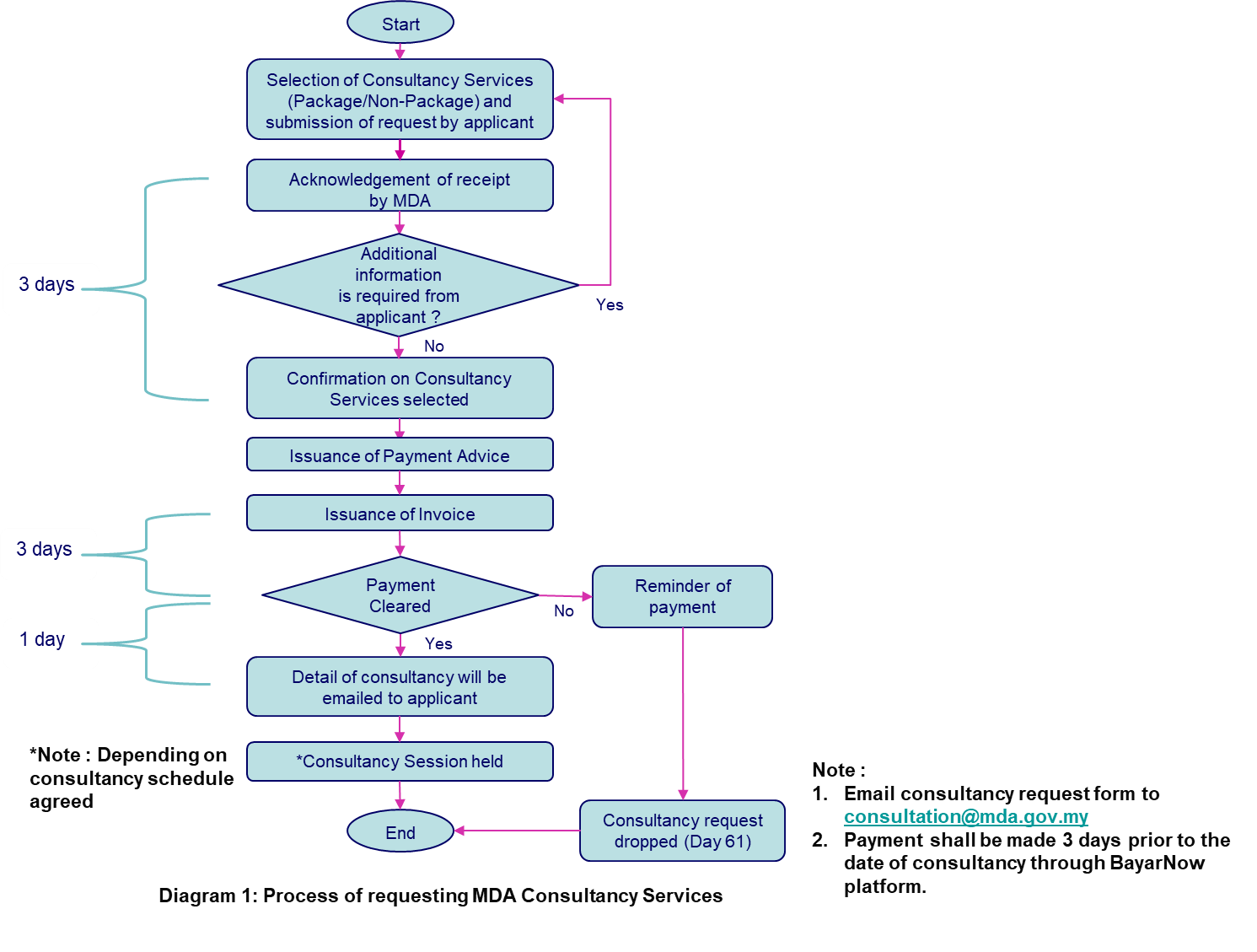
If you want to know more about our consultancy services, send an email to consultation@mda.gov.my
Updated: 21 August 2023






